

| Line spectrum is the discrete component in the spectrum, which is emitted from the celestial objects and shown as a function of wavelength (or frequency or photon energy). That is, at some wavelength, the intensity of radiation is strong or weak, compared with the continuous component. |
 | |
|
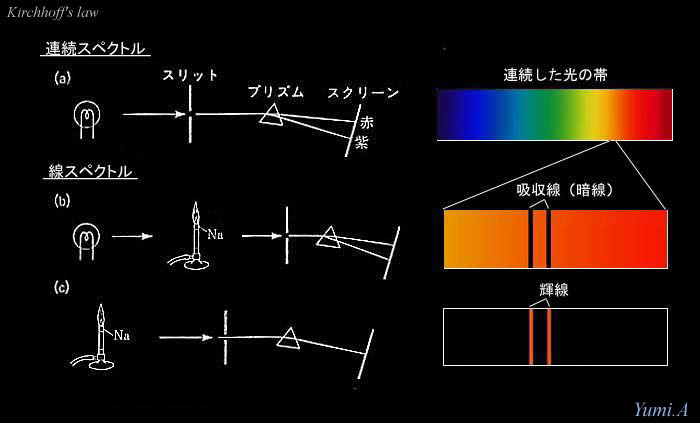
(a)
When we resolve the light from the electric bulb (lamp)
by the prism,
it is decomposed into the continuous spectrum
(continuum) on the screen.
(b) If sodium (Na) burns between the bulb and prism, there appears dark lines in the continuum. This is because the sodium atom absorbs radiation at some specified wavelengths in the continuous radiation from the lamp. Such a dark line is called absorption line.
(c)
Furthermore,
the light from the burning sodium emitts
only the radiation at some specified wavelengths,
which is equal to those of the absorption lines.
This is because the sodium atom
emitts radiation with same wavelengths.
Such an emission at some wavelengths is called
emission line.
|
These emission and absorption lines are
generally called line spectrum.
Detailed speaking,
the solar spectrum has many fine dark lines
(Fraunhofer lines).
|
 Go to Submenu
Go to Submenu Go to Menu
Go to Menu
自然科学書出版 裳華房 SHOKABO Co., Ltd.