

| Radiation is produced via various processes (radiation processes). Emission mechanism of line spectrum includes atomic and molecular lines. |
Atomic line |
Recombination line
Atom has discrete energy states (energy level).
When atom transits from a high energy level to a low energy level,
it radiates line emission with some specified wavelength,
corresponding to the energy difference between both levels.
|
||||||||||||||||||||
Vibrational transition of CO Rotational transition of CO 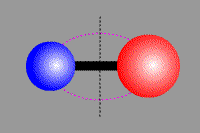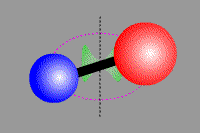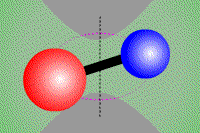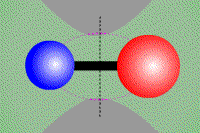
Vibrational transition of H2O |
(Vibrational transition) (Rotational transition) Molecules, which consist of multiple atoms, can vibrate or rotate around an axis. When a vibrational or rotational state of molecules discretely changes, obeying quantum dynamics, they emit or absorb line radiation. Electron energy transition as well as vibrational and rotational transitions make moleclar lines. Cabon monoxide CO, which is a typical two-atom molecule, consists of cabon atom (●) and oxigen atom (●). This carbon monoxide can vibrate in the direction of a combined axis, while can rotate around the axis.
In order to express such vibrational or rotational transition,
we use
|
||||||||||||||||||||
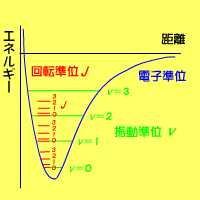
|
Energy level for two-atom molecules. A blue curve expresses the electron energy level in the fundamental state, green bars means the vibrational energy level, and red bars denotes the rotational energy level. | ||||||||||||||||||||
Reverse transition of NH3 |
(Reverse transition)
A three-dimensional molecule like ammonia
emits electromagnetic radiation,
by reversing its configuration.
|
||||||||||||||||||||
 Go to Submenu
Go to Submenu Go to Menu
Go to Menu
自然科学書出版 裳華房 SHOKABO Co., Ltd.